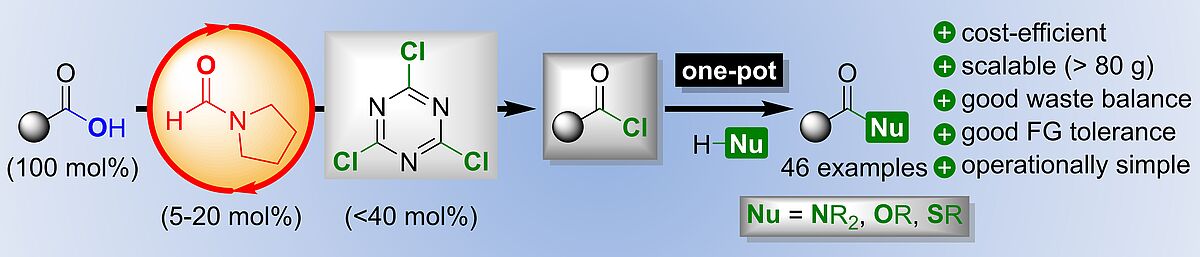
Research topics
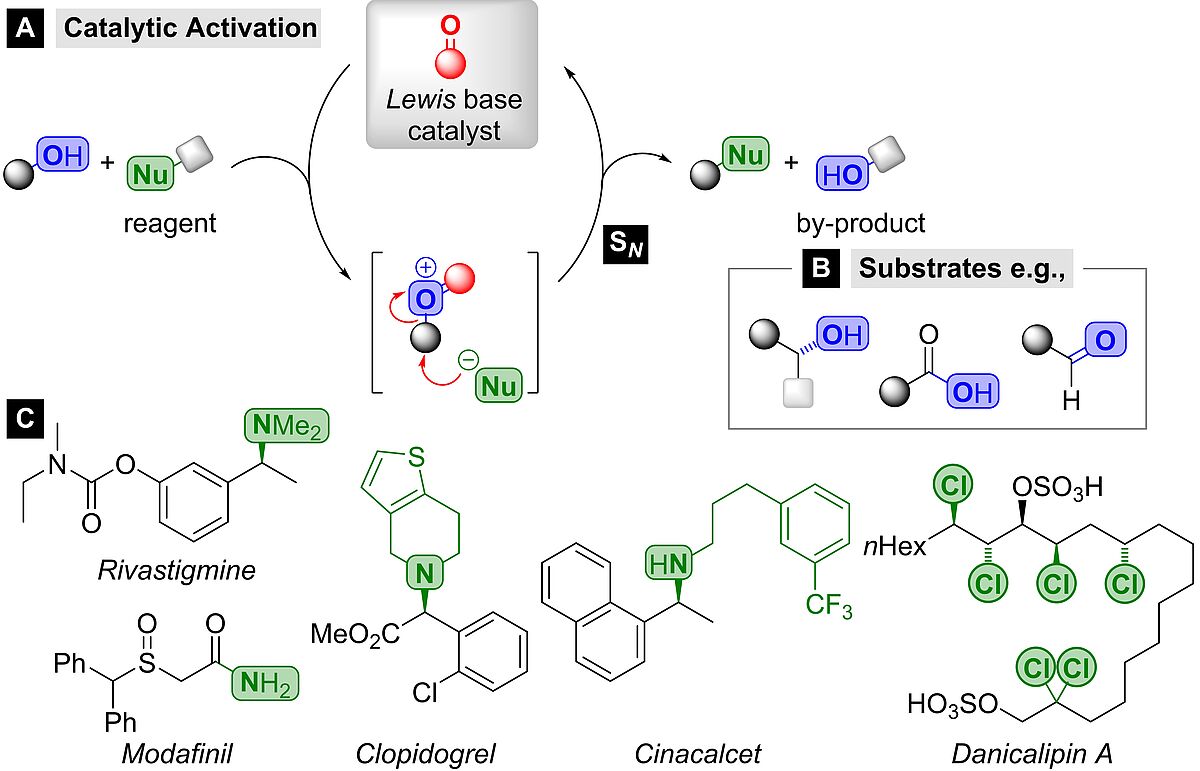
Nucleophilic substitution (SN) reactions account to the most essential and frequently applied transformations in chemistry. Nevertheless, they are typically associated inevitably with the generation of unfavourable waste amounts and are therefore restricted by a poor sustainability and a poor cost-efficiency. To address these major challenges, we are committed towards the development of novel catalytic methods for SN-type bond formations. In that regard, we are particularly interested in:
- Homogeneous catalysis, especially Lewis-base- and organocatalysis
- Sustainable chemistry
- Transition-metal catalysed carbonylative transformations
- Synthesis of pharmaceuticals and natural products
- photocatalysis
You can find more information about our research at peterhuylab.de
Funding
For generous support, we would like to acknowledge the Fonds of the Chemical Industry (Liebig fellowship), the German Research Foundation (DFG) and the University of Rostock.
Recent Publications
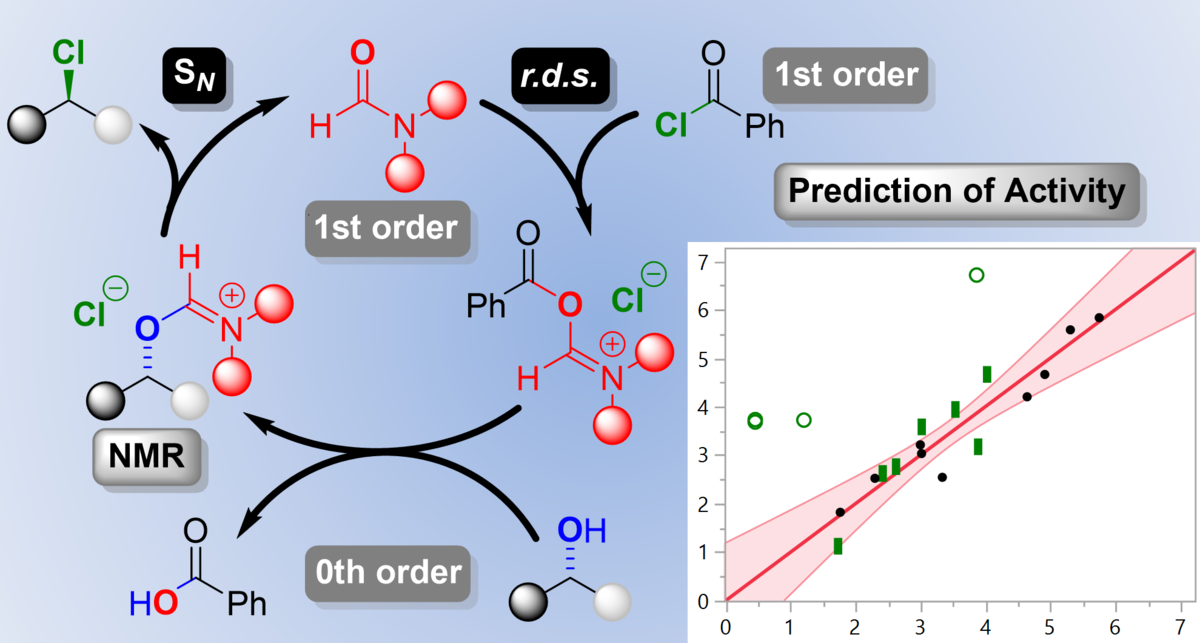
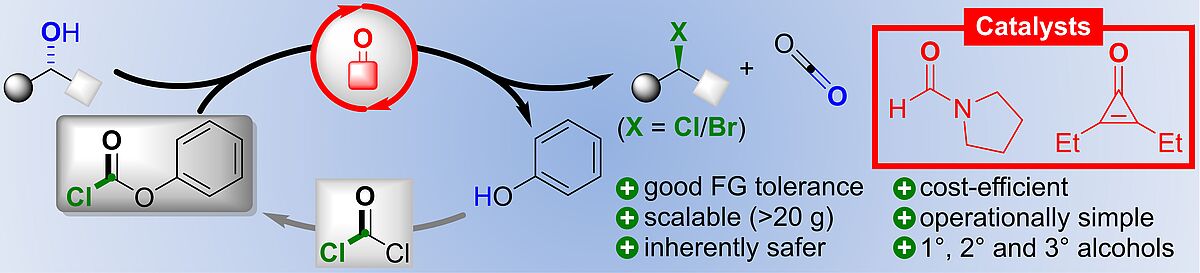
B. Zoller, T. Stach, P. H. Huy,* "Lewis Base Catalysis Enables the Activation of Alcohols by means of Chloroformates as Phosgene Substitutes", ChemCatChem 2020, doi: 10.1002/cctc.202001175.
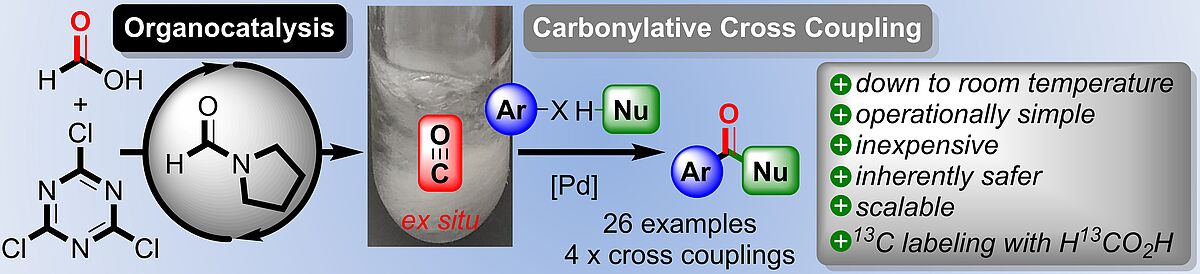
B. Zoller, J. Zapp, P. H. Huy,* "Rapid Organocatalytic Formation of Carbon Monoxide: Application towards Carbonylative Cross Couplings", Chem. Eur. J. 2020, 226, 9632-9638.